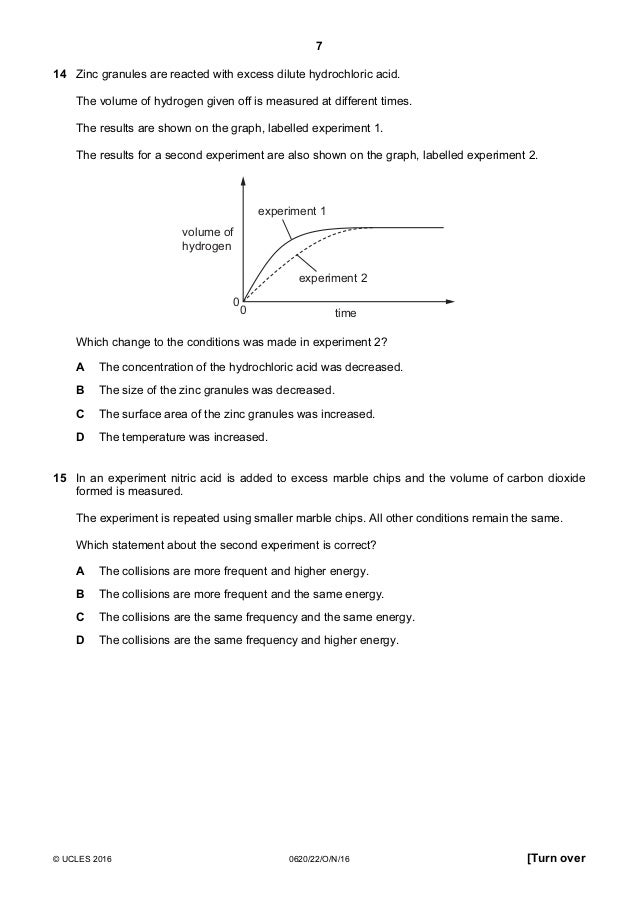
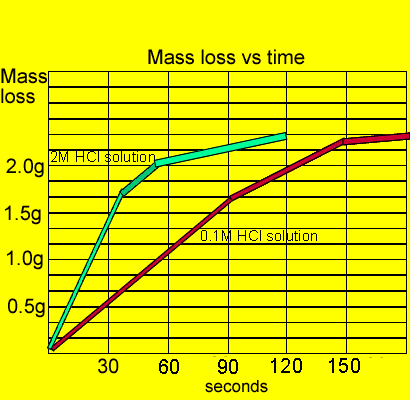
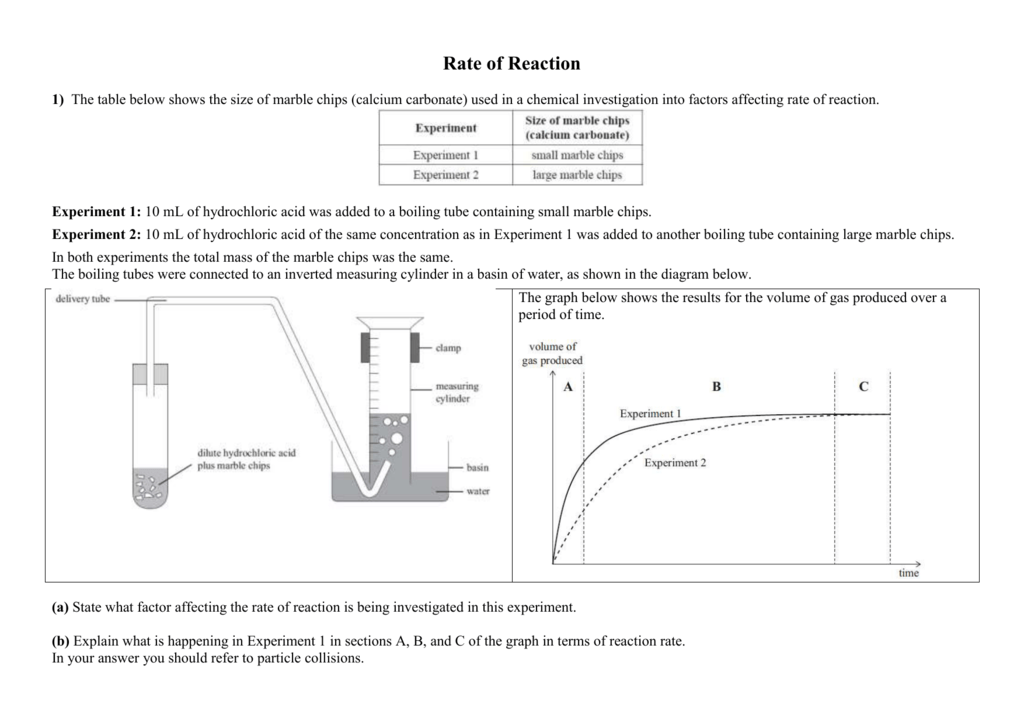
Marble Chips And Hydrochloric Acid Experiment Graph

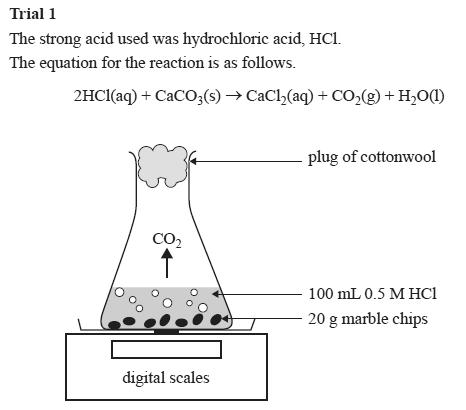
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
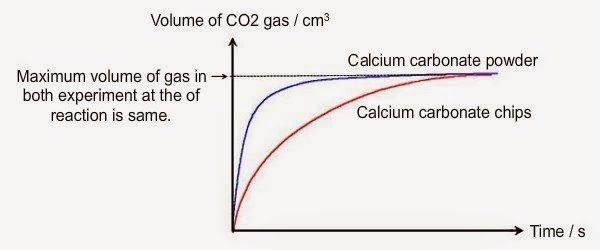
Marble chips and hydrochloric acid experiment graph. Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas. Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim. Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method. There are many variables that affect.
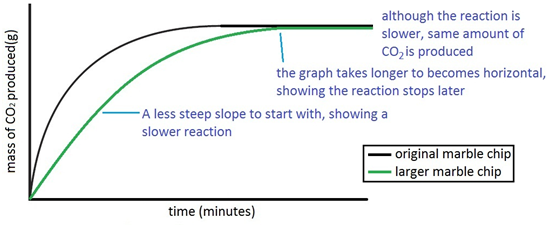
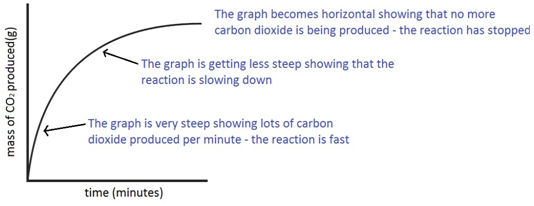
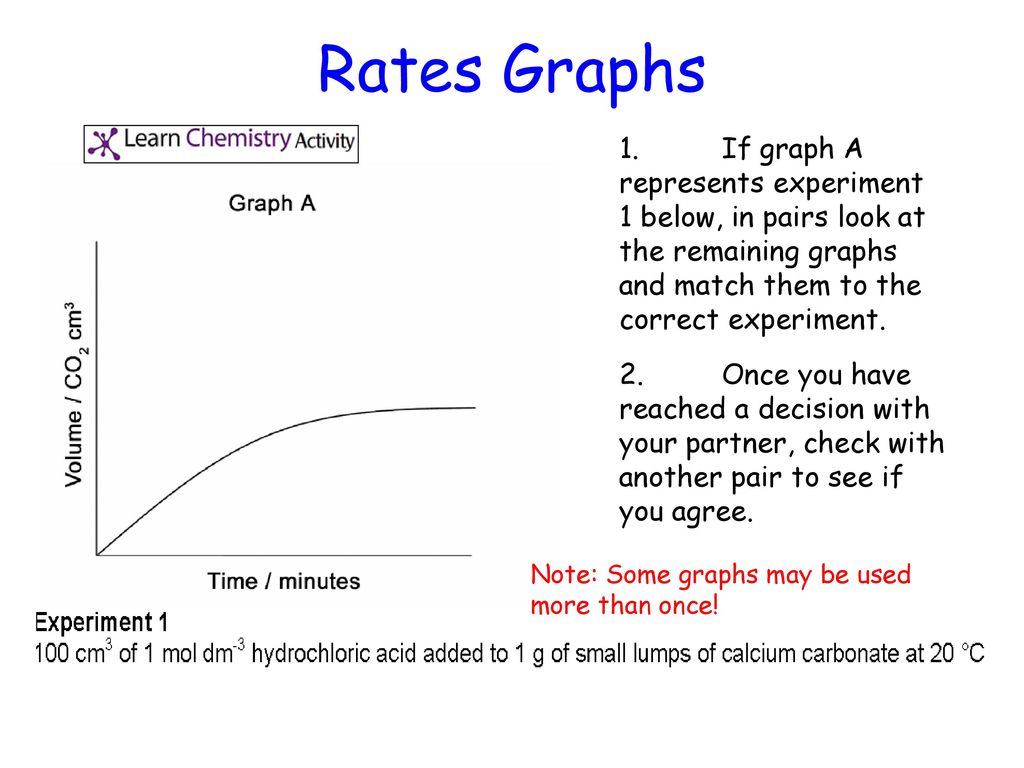
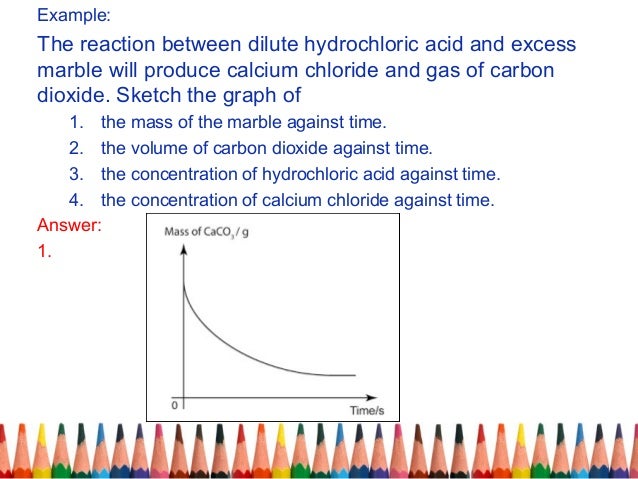
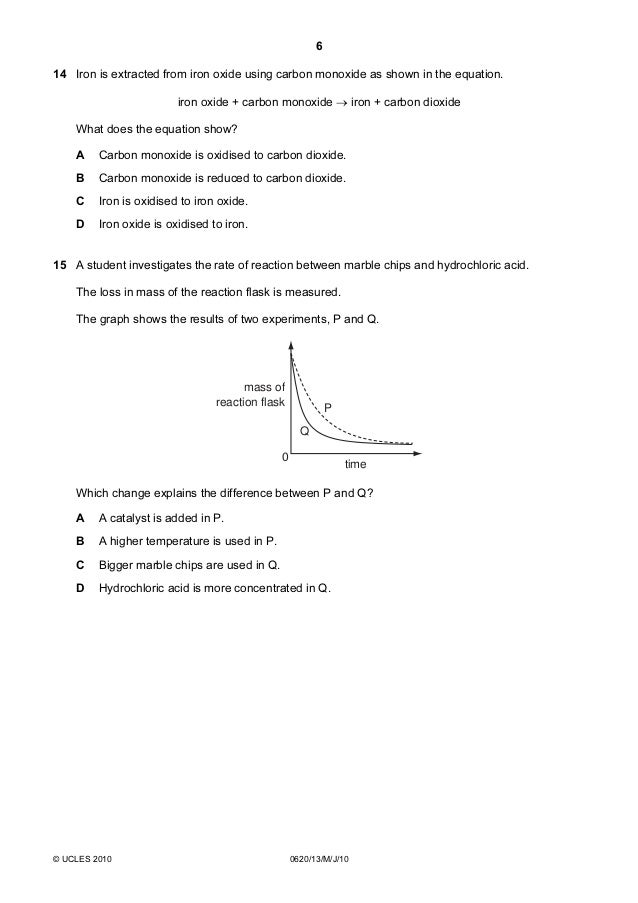
An investigation of the reaction between marble chips and hydrochloric acid. The curve on the graph goes flat when the reaction is complete. Calcium chloride solution is also formed. Measuring the rate of loss of a gaseous product.
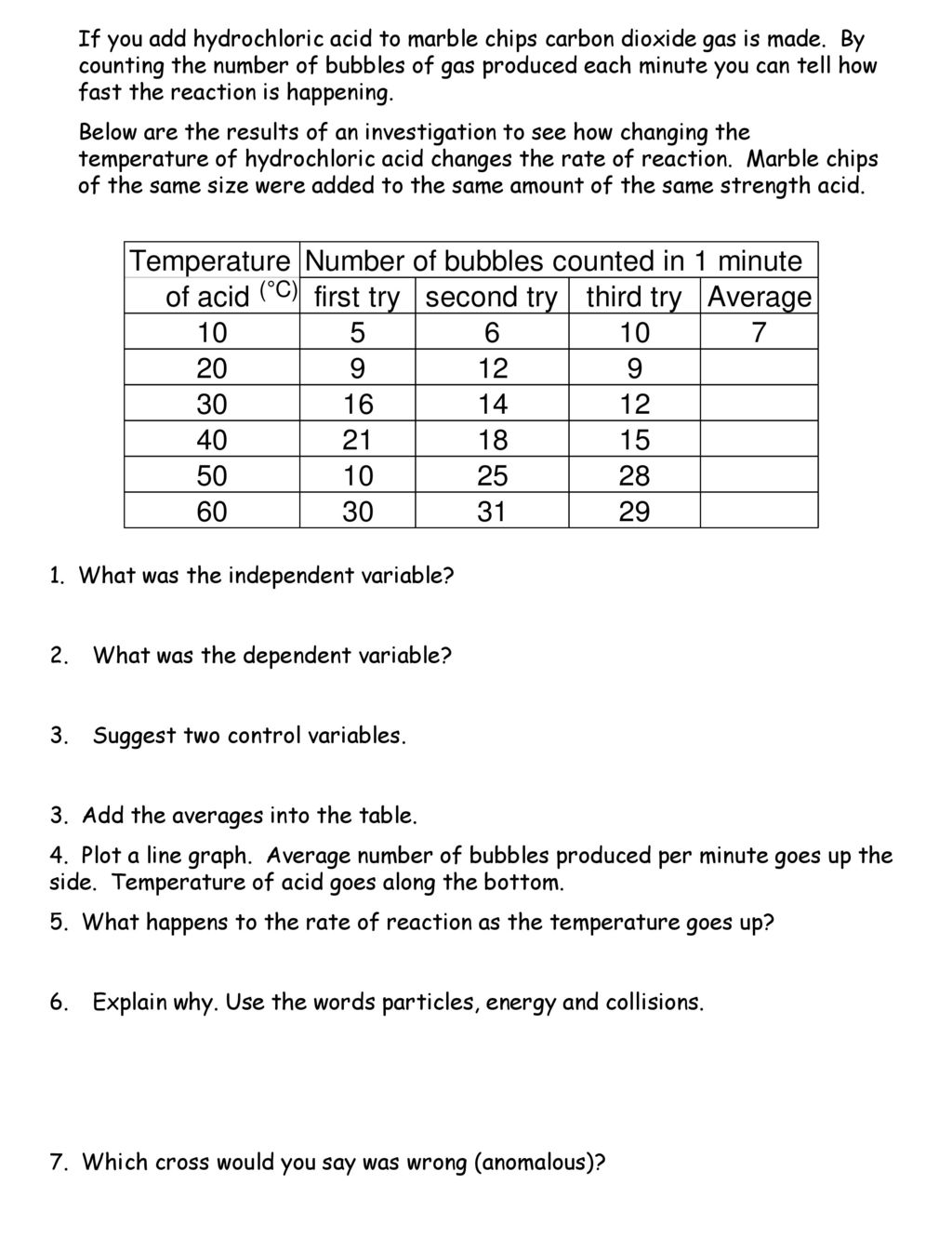
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute. Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place. This is because as time goes on the volume of the gas evolved does not change. Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
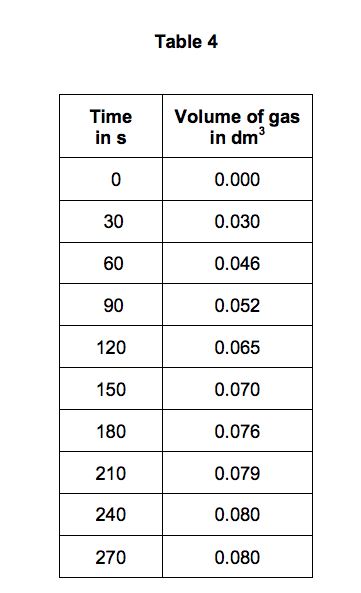
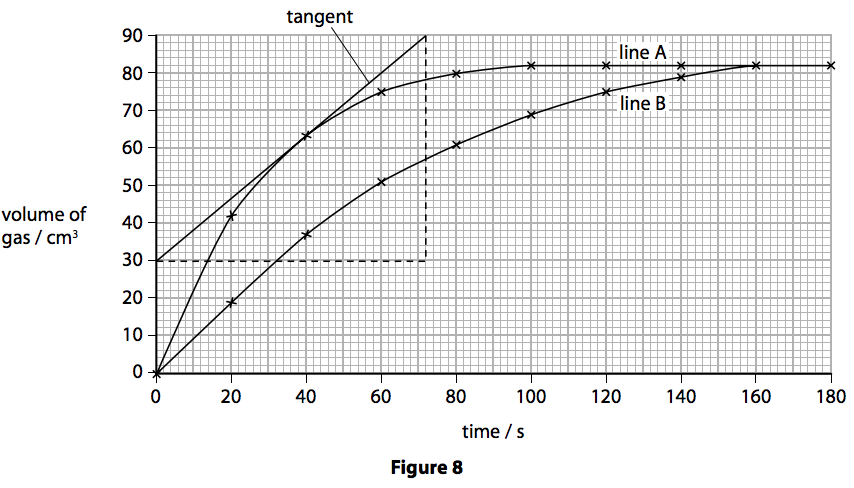












.jpg)